Meeting the unmet need in RAS-driven cancers
Our pipeline is developing RAS-directed oral agents for RAS-mutated cancers.

Our pipeline is developing RAS-directed oral agents for RAS-mutated cancers.
|
|
|
|
|
|
|
|
|
|
|
|
We are advancing three oral small molecule inhibitors of RAS signaling with differing RAS targets. YL-17231 is being investigated in two Phase1 studies in cancer patients with tumors having any KRAS, NRAS and HRAS alterations. YL-15293 is a covalent KRASG12C-specific oral inhibitor in Phase 1 for patients with KRASG12C tumors. YL-17424, declared to be a Development Candidate in 2024, is in IND-enabling steps. YL-17424 is a KRASmulti with potent activity against tumors that are KRASG12D, V, C and KRASwildtype-amplified.
Key residues in the KRAS oncoprotein (KRASG12D, KRASG12V, KRASG12C, and other KRAS and NRAS mutations) are mutated in cancers, at nearly 250,000 new cases and one-quarter of all cases in the U.S. alone.
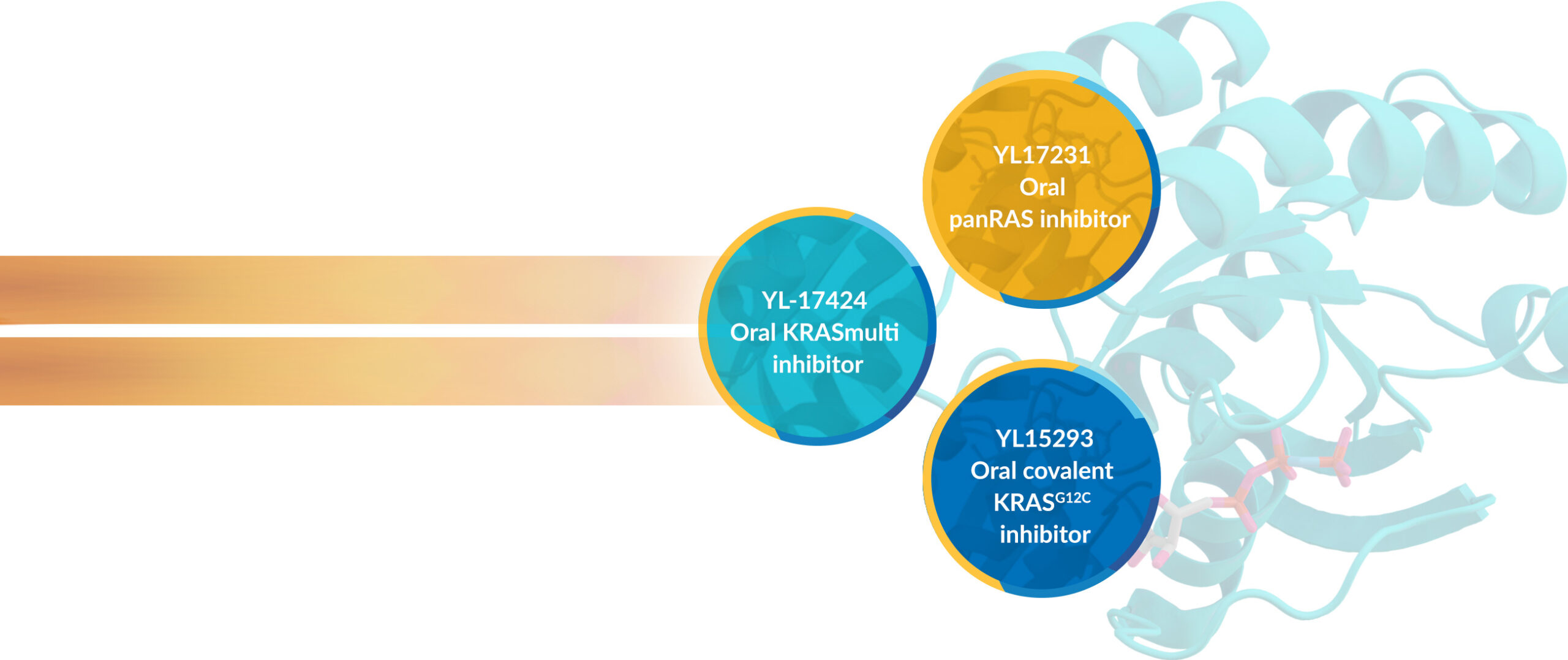
Key residues in the KRAS oncoprotein (KRASG12D, KRASG12V, KRASG12C, and other KRAS and NRAS mutations) are mutated in cancers, at nearly 250,000 new cases and one-quarter of all cases in the U.S. alone.